The Desmin Gene
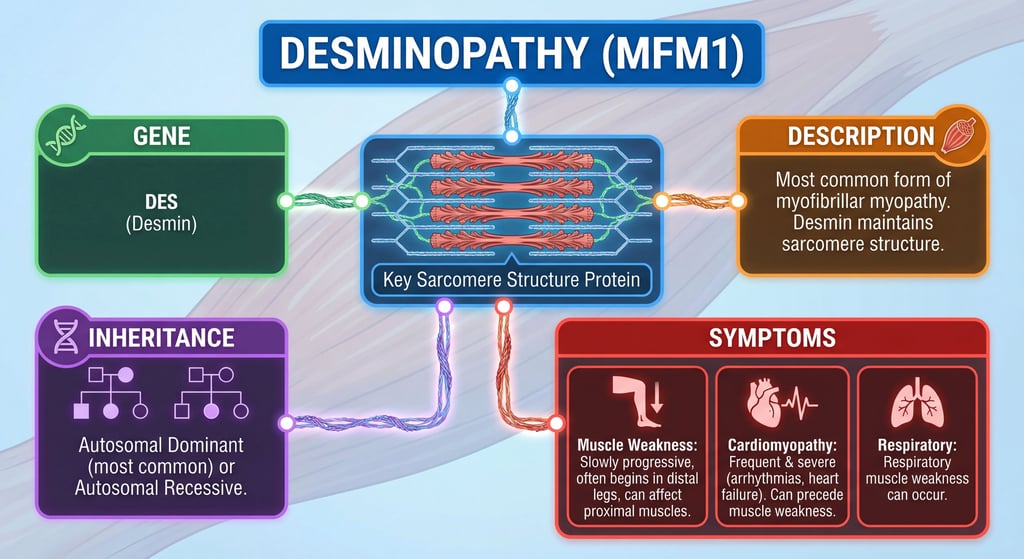
- The desmin gene, officially known as DES, is located on chromosome 2q35 and encodes the desmin protein, a key component of the muscle cell cytoskeleton.
- Desmin forms intermediate filaments that help maintain muscle structure and function, particularly in heart, skeletal, and smooth muscles.
- Mutations in this gene are linked to desminopathy, a rare muscle disorder involving protein buildup and muscle degeneration.
Function and Structure
The DES gene produces desmin, a 53.5 kDa protein made of 470 amino acids. It belongs to the type III intermediate filament family and is muscle-specific. Desmin's structure includes a central alpha-helical rod domain flanked by non-helical head and tail domains, enabling it to form filamentous networks. These networks connect myofibrils at Z-disks to the cell membrane, nucleus, and organelles like mitochondria, supporting muscle contraction, integrity, and force transmission. High expression occurs in heart and skeletal muscle, with roles in cytoskeleton organization and heart contraction regulation.
What is Desminopathy?
Desminopathy, also called desmin-related myofibrillar myopathy, is a rare genetic condition caused by DES gene mutations. It leads to abnormal protein aggregates in muscle cells, disrupting their structure and function. Symptoms often include progressive weakness starting in the legs, heart problems like arrhythmias, and sometimes breathing difficulties. Onset varies from childhood to adulthood, and while there's no cure, management focuses on symptoms like pacemakers for heart issues.
Causes and Inheritance
Mutations in DES, such as amino acid substitutions or deletions, impair desmin's ability to form proper filaments, causing muscle damage. It's usually inherited in an autosomal dominant pattern, meaning one faulty gene copy can cause the disease, but recessive forms exist. De novo mutations can occur without family history.
---
Desmin, encoded by the DES gene on human chromosome 2q35, represents a cornerstone of muscle cell architecture as a type III intermediate filament protein. This gene spans approximately 8.4 kb and comprises 9 exons, producing a 470-amino-acid protein weighing 53.5 kDa. The protein's structure is tripartite: a conserved alpha-helical rod domain (308 residues) flanked by globular N-terminal (head) and C-terminal (tail) domains, facilitating self-assembly into 10-nm filaments. Desmin is predominantly expressed in cardiac, skeletal, and smooth muscle tissues, with biased expression in the heart (RPKM 2605.8) and esophagus (RPKM 953.7), among others. Its filaments interconnect myofibrils at Z-disks, link them to the sarcolemma via costameres, and anchor to organelles like mitochondria and the nucleus, ensuring mechanical stability, force transmission, and proper subcellular organization during muscle contraction. Phosphorylation sites in the head domain, modulated by kinases like Cdc2, regulate filament dynamics, while interactions with proteins such as myospryn, desmoplakin, and alphaB-crystallin extend its roles in lysosome biogenesis and cellular signaling.
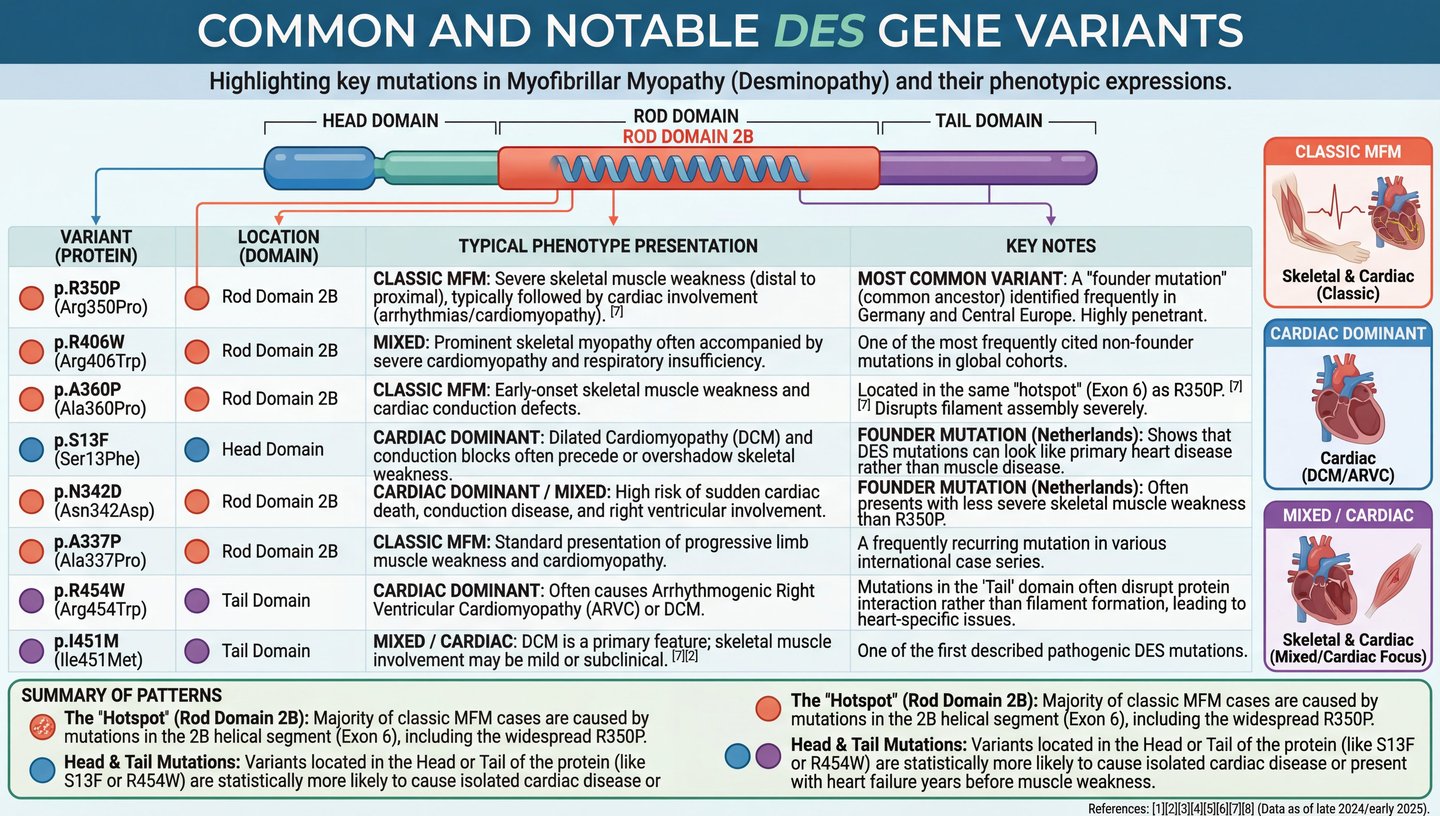
Desminopathy, synonymous with desmin-related myofibrillar myopathy, emerges from pathogenic variants in the DES gene, manifesting as a heterogeneous protein aggregate myopathy within the broader category of myofibrillar myopathies (MFMs). This rare disorder, with unknown prevalence but estimated at ≤5/10,000, features abnormal chimeric aggregates of desmin and other cytoskeletal proteins, alongside granulofilamentous material visible ultrastructurally in muscle biopsies. Etiologically, over 67 mutations have been identified, predominantly missense variants clustering in the coil 2B domain of the rod, though head and tail mutations are noted, often linked to cardiac predominance. Inheritance is primarily autosomal dominant, with recessive forms in about 5 families, and sporadic de novo cases contributing to variability. These mutations disrupt filament assembly, promote aggregation, impair protein quality control (e.g., ubiquitin-proteasome and autophagy pathways), and induce mitochondrial dysfunction, including complex I inhibition and oxidative stress.
Clinically, desminopathy presents with bilateral skeletal muscle weakness, initiating distally in the legs and progressing proximally to involve trunk, neck flexors, facial muscles, and occasionally bulbar functions. Onset spans infancy to late adulthood, with recessive variants often emerging in childhood and dominant ones in the second to fourth decades. Cardiac involvement affects 74% of cases, encompassing dilated, restrictive, hypertrophic, or arrhythmogenic right ventricular cardiomyopathy, conduction blocks (e.g., AV block, bundle branch blocks), arrhythmias, and heart failure. Respiratory insufficiency, starting with nocturnal hypoventilation and advancing to daytime failure, occurs in 26%, often necessitating ventilation support. Additional manifestations include cataracts, swallowing difficulties, intestinal issues, and muscle stiffness or cramps. Progression is inexorable, leading to wheelchair dependence and mortality around age 49 from cardiac or respiratory complications in about 26% of reported cases.
Diagnosis relies on clinical evaluation, elevated creatine kinase (often mild, ≤4-fold normal), electromyography showing myopathic patterns with irritative features, and muscle biopsy revealing myofibrillar degeneration, desmin-positive aggregates, and Z-disc streaming. Genetic sequencing confirms DES mutations, while cardiac assessments (ECG, echocardiography, Holter monitoring, MRI) detect subclinical involvement. Differential diagnoses include other MFMs (e.g., due to BAG3, FLNC, or CRYAB mutations) and laminopathies. Genetic counseling emphasizes 50% transmission risk in dominant forms and carrier testing for recessive variants.
Management is supportive, lacking curative options. Cardiac interventions include pacemakers, implantable cardioverter-defibrillators, and transplantation for severe cardiomyopathy. Respiratory therapy, physical rehabilitation, orthotics for foot drop, and annual screenings mitigate progression. Investigational approaches, such as metformin for related MFMs or autophagy enhancers, hold promise but require further trials.
Animal models, including desmin knockout mice, recapitulate phenotypes with myofibrillar disorganization, mitochondrial abnormalities, and reduced muscle resilience, underscoring desmin's non-essential role in myogenesis but criticality for maintenance. Transgenic models with mutations like R173_E179del or L345P reveal aggregate formation, conduction defects, and therapeutic targets like Bcl-2 overexpression to ameliorate mitochondrial defects.
Future research should integrate filament biomechanics, epigenetics, and organelle interactions to unravel phenotypic variability and develop targeted therapies, such as siRNA or chaperone modulators
Epidemiology and Prognosis Table
| Aspect | Details |
| Prevalence | Unknown; rare (≤5/10,000), ~2% of dilated cardiomyopathies |
| Age of Onset | Variable: Childhood (recessive), 20-40 years (dominant) |
| Gender Bias | Males more prone to cardiac manifestations |
| Mortality Rate | ~26%, mean age 49 years; causes include sudden cardiac death, heart failure, respiratory failure |
| Progression | Slow to moderate; leads to wheelchair dependence in many cases |